Translations may contain older or less detailed information than the original German page!
Diagnosis

Summary:
The most difficult part of the diagnosis is probably to develop an initial suspicion that it probably could be a mast cell activation disease (MCAD) and to consider this as a possible diagnosis in the procedure of differential diagnosis.
Choice of doctor
The necessary clarifications for the diagnosis of MCAD can be made or commissioned by any family practice. Because of the overload of specialized doctors, it makes sense when the family doctors take the lead as far as possible. For specific questions or in difficult cases, the nearest center of excellence for mast cell disease can be contacted, or the patient will be transferred there.
Addresses of MCAD centers of excellence in Europe
Mast cells originate in the bone marrow and are counted among the blood cells (although they do not occur in the blood). Hematologists are specialized in blood and blood-forming organs (bone marrow). Not all allergists are familiar with MCAD. But allergists are usually competent specialists regarding the differential diagnosis (clarification of other incompatibilities, allergy screening) and can therefore also be visited for an initial assessment.
Diagnostic criteria for MCAD
Diagnostic definition of mast cell activation (MCA)
If all of these three criteria are met, it can be assumed that one is dealing with mast cell activation (MCA) [Valent et al. 2012, S. 215; Brockow 2013; Molderings et al. 2014]:
- Typical symptoms are present, not otherwise explainable and in need of treatment.
- Elevated concentrations of mediators or their degradation products are detectable
- Response to treatment with mast cell specific drugs
Difficulties:
Criterion 1: The symptoms alone are non-specific and therefore not conclusive.
Criterion 2: The detection of mast cell mediators (or their degradation products) does not succeed very often, for the reasons explained below. Therefore, the criterion 2 must in practice most often be dropped and is not a criterion for exclusion.
Criterion 3: The response to drugs may be hampered because of diarrhea (intravenous administration may then be attempted), because only the healthy, secondarily activated mast cells can be influenced by therapy, but not those who are pathologically altered. Another reason may be that unsuitable products with incompatible active substances or incompatible adjuvants were chosen, or in rare cases also due to an allergy to an ingredient.
Subsequently, by means of differential diagnosis, it must be clarified whether the mast cell activation is caused by a MCAD, or if it could be of secondary origin (e.g. allergies, inflammatory diseases).
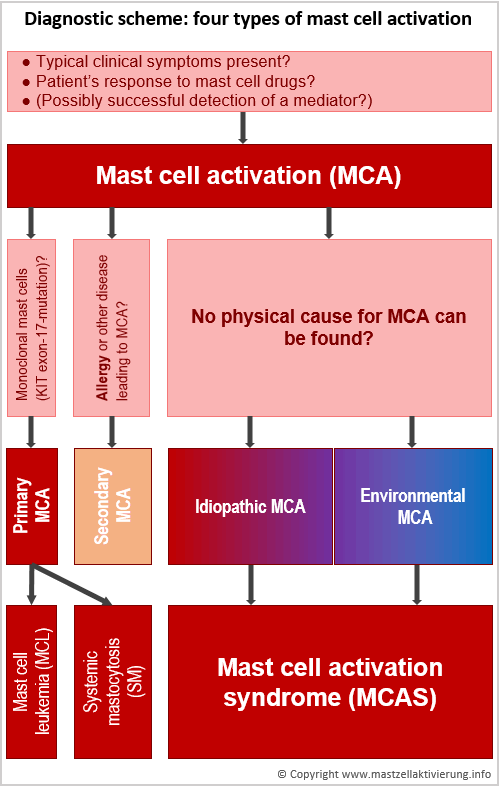
It can be distinguished between primary, secondary, idiopathic and environmental mast cell activation:
- Primary: forms of mastocytosis with known physical cause.
- Secondary: other diseases with mast cell activation, for example, allergies
- Idiopathic: unknown cause. Probably also counting for mastocytosis, although the current WHO diagnostic criteria are not met.
- Environmental: activation due to unfavorable external, non-physical causes. The mast cells of every healthy person can be activated as well, if stimulus intensity is strong enough.
Diagnostic criteria for MCAS and mastocytosis
Proposed diagnostic criteria for the mast cell activation syndrome (MCAS), and the official WHO criteria for the diagnosis of systemic mastocytosis (SM), based on Molderings et. al. [2011, Tab. 2]:
| Criteria for the mast cell activation syndrome (MCAS) | WHO criteria for systemic mastocytosis (SM) |
|---|---|
| The diagnosis MCAS is made when both major criteria or the second main criterion and at least one minor criterion are met. | The diagnosis SM can be established if the main criterion and at least one minor criterion or at least three minor criteria are met. |
| Main criteria | Main Criterion |
| Dense infiltrates of mast cells, scattered or in clusters, in biopsies from bone marrow or other internal organs / tissues (not the skin), stained with CD117, tryptase and CD25. | Several dense mast cell infiltrates (> 15 mast cells per collection) in biopsies from bone marrow or other internal organs / tissues (not the skin), stained with CD117, tryptase and CD25. |
| Existing symptomatology suggests an increased mast cell activity (mast cell mediator syndrome) | |
| Minor criteria | Minor criteria |
| More than 25% of the mast cells in the biopsy (smear or histology) have an atypical shape (fusiform or oval instead of round). | More than 25% of the mast cells in the biopsy (smear or histology) have an atypical shape (fusiform or oval instead of round). |
| Mast cells in bone marrow expressing CD2 and / or CD25 | Mast cells in bone marrow expressing CD2 and / or CD25 |
| Detection of genetic alterations in mast cells from blood, bone marrow or internal organs, which are known to increase the mast cell activity | KIT D816V mutation in mast cells from internal organs / tissues |
| Detection of a pathologically increased release of mast cell mediators (relative to the base level in the symptom-poor phase) at determination of tryptase in the blood, N-methylhistamine in urine, heparine in the blood, chromogranine A in the blood, or of other mast cell-specific mediators (e.g., leukotrienes, prostaglandine D2) | Total serum tryptase> 20 ng / ml (not applicable with the simultaneous appearance of a non-mast cell blood disease) |
No exclusion criteria
The WHO criteria are not absolute diagnostic criteria, but an interim classification system with specific inclusion criteria. However, they are not sufficiently sensitive and specific. If the criteria are not met, this should not lead to exclude an MCAD. [Homann et al. 2010b]
Diagnostic procedure
In broad outline, the procedure might look like this:
1. Anamnesis (case history interview )
The physician should always be aware of the following difficulties:

- The clinical picture is not uniform. It may present itself totally different in every case. It is as changeable as a chameleon.
- Patient's histories or experience reports are usually incomplete. The doctor sees only the tip of the iceberg.
- The triggers are difficult to identify. Incompatible food is often mistaken for tolerated.
- A mindset of combating just the symptoms dominates, instead to aim at a holistic understanding of the system. There are merely given medicines for individual symptoms, rather than to understand the cause and to treat it as specifically as possible.
- Many diagnoses, but lack of therapeutic success are typical. Often single mast cell mediator symptoms or secondary diseases (sequelae) are mistaken for the actual disease, without seeing the mast cell disease as the underlying cause (e.g. "irritable bowel syndrome", "non-allergic rhinitis", "urticaria", "chronic fatigue syndrome", "sleeping disorder", "psychosomatics"). A flood of diagnoses and treatment failure in the past should therefore let the doctor think of an MCAD.
- Contrary to previous expert opinion MCAD are not rare, but affect a double-digit percentage of the population! Moreover, persons concerned are probably seeing a physician more often than the average population. The probability might therefore be very high that a MCAD is the actual (but unrecognised) reason for a medical consultation.
- Mastocytosis is not a pure skin disease. Any organs or organ systems can be affected in different constellation, with or without skin involvement
2. Differential diagnosis
The differential diagnosis (clarification of other diseases that may also be responsible for the observed symptoms) is a complicated detective game that we can not represent comprehensively here by a simple scheme. The procedure is very dependent on the individual case.
If the symptoms are triggered by several physical causes, treatment of only one of these diseases will not be successful. Don't forget to consider the possibility of a seronegative gastrointestinal allergy, which is undetectable by means of prick or blood test.
3. Laboratory Diagnostics
The diagnosis of MCAD will at least in the foreseeable future primarily be based on laboratory tests to detect elevated levels of mast cell-specific mediators or their breakdown products (metabolites) in blood and urine. However, these as well as the other methods described here have their weaknesses.
Mediator detection
Evidence of the many liberated mast cell mediators or their degradation products fail very often or even most. That is because they bind to receptors or are degraded before they get into the bloodstream, or for many other reasons. This leads to two conclusions:
- Only a positive result can be conclusive. A negative result can not exclude the existence of a MCAD.
- The detected concentrations do not allow conclusions about the severity of the disease or on the intensity of the symptoms [Broesby-Olsen S et al. 2013].
To confirm the diagnosis and for the subclassification of a MCAD the determination of tryptase and heparin in blood and methylhistamine in the urine is recommended. In the future, other biomarkers should be included as soon as these will be available in the commercial routine diagnostics. [Molderings 2014]
Gastroscopy, colonoscopy, tissue samples
Since almost always the digestive tract is concerned as well, a gastroscopy and colonoscopy is recommended for a more accurate evaluation. These studies are also used for removal of tissue samples (biopsies) in order to find noticeable arrangements and shapes (morphology) of the mast cells and increased mast cell density. Only positive biopsy findings are conclusive. Negative results should not be seen as an exclusion criterion.
Mutation analysis
Since MCAD are caused by genetic mutations in mast cells, it is natural to seek for different gene variants by means of genetic tests or mutation analysis. Our knowledge, however, is still very incomplete do determine how relevant each possible combination of several acquired gene defects affecting several genes is. As in the tissue sample only a few single cells show the acquired mutation, such mutation analysis is methodologically more complex than a common genetic test, where a mutation can be found in every cell of the entire body. Currently, only the specific PCR for the mutation KIT D816V is routinely available.
Bone marrow biopsy
The examination of a tissue sample from the pelvis bone marrow can be used as an additional diagnostic criterion. It can draw the attention to any existing additional haematological disease (disease of blood cells) or it can show an excessive migration of mutated mast cells. It is applied at most if the other diagnostic criteria indicated a suspected systemic mastocytosis (basal tryptase level >20 ng/ml; morphologically abnormal mast cells in the biopsies from the gastrointestinal tract). False negative results are common, because with the biopsy often none of the spread nest with mutant mast cells is hit. Only a positive finding can be conclusive.
Tentative implementation of the therapy
The diagnostic methods listed above provide a positive test result only in a small proportion of cases. Most persons concerned with MCAD would fall through the cracks without getting a diagnosis (false negative result). Not only the tested positive but especially also the tested negative, should therefore undergo a further diagnostic step: The tentative (experimental) performing of a mast-cell-specific therapy (avoid triggers, medication) for a limited period of time. For details see page Therapy and subpages. For doctors, it may perhaps be unfamiliar and unusual to start therapy before an iron-clad diagnosis exists. However, in the absence of better alternatives this is the most sensible and effective one can do in this case.
If the patient is not responding to the medication and this could be the case because of diarrhea, medication may be administered intravenously where possible.
Find a doctor outside Europe (international, USA, Canada)
Find an allergist / immunologist recommended by AAAAI (but not necessarily specialised in mast cell disorders): allergist.aaaai.org/find
Recommended literature for the diagnosis of MCAD
| Literature for the diagnosis of MCAD | |
|---|---|
| Afrin et al. 2015 | Afrin LB, Pöhlau D, Raithel M, Haenisch B, Dumoulin FL, Homann J, Mauer UM, Harzer S, Molderings GJ.: "Mast Cell Activation Disease: An Underappreciated Cause of Neurologic and Psychiatric Symptoms and Diseases." Brain Behav Immun. 2015 Jul 7. pii: S0889-1591(15)00236-6. doi: 10.1016/j.bbi.2015.07.002. https://pubmed.ncbi.nlm.nih.gov/26162709 (Focus on neurological and psychiatric symptoms and sequelae of MCAD. "We describe MCAD's pathogenesis, presentation (focusing on [central and/or peripheral neurologic and/or psychiatric symptoms] (NPS)), and therapy, especially vis-à-vis neuropsychotropes. Since MCAD patients often present NPS, neurologists and psychiatrists have the opportunity, in recognizing the diagnostic possibility of MCAD, to short-circuit the often decades-long delay in establishing the correct diagnosis required to identify optimal therapy.") |
| Molderings et al. 2014 | Molderings GJ, Homann J, Brettner S, Raithel M, Frieling T: "Systemische Mastzellaktivierungserkrankung: Ein praxisorientierter Leitfaden zu Diagnostik und Therapie" [Mast cell activation disease: a concise practical guide for diagnostic workup and therapeutic options]. Dtsch Med Wochenschr. 2014 Jul;139(30):1523-34; quiz 1535-8. doi: 10.1055/s-0034-1370055. Epub 2014 May 6. http://www.ncbi.nlm.nih.gov/pubmed/24801454 Very good and current overview article. |
| Valent et al. 2012 | Valent P, Akin C, Arock M, Brockow K, Butterfield JH, Carter MC, Castells M, Escribano L, Hartmann K, Lieberman P, Nedoszytko B, Orfao A, Schwartz LB, Sotlar K, Sperr WR, Triggiani M, Valenta R, Horny HP, Metcalfe DD.: "Definitions, criteria and global classification of mast cell disorders with special reference to mast cell activation syndromes: a consensus proposal". Int Arch Allergy Immunol. 2012;157(3):215-25. Epub 2011 Oct 27. http://www.ncbi.nlm.nih.gov/pubmed/22041891 Freely accessible overview article, but not the most recent. (Propose a global unifying classification of all MC disorders and pathologic MC reactions. This classification includes three types of 'MCA syndromes' (MCASs), namely primary MCAS, secondary MCAS and idiopathic MCAS. MCA is now defined by robust and generally applicable criteria, including (1) typical clinical symptoms, (2) a substantial transient increase in serum total tryptase level or an increase in other MC-derived mediators, such as histamine or prostaglandin D(2), or their urinary metabolites, and (3) a response of clinical symptoms to agents that attenuate the production or activities of MC mediators.) |
| Molderings et al. 2011 | Molderings GJ, Brettner S, Homann J, Afrin LB.: "Mast cell activation disease: a concise practical guide for diagnostic workup and therapeutic options". J Hematol Oncol. 2011 Mar 22;4:10. http://www.ncbi.nlm.nih.gov/pubmed/21418662 Freely accessible overview article, but not the most recent. |
| Hamilton et al. 2011 | Hamilton MJ, Hornick JL, Akin C, Castells MC, Greenberger NJ.: "Mast cell activation syndrome: a newly recognized disorder with systemic clinical manifestations." J Allergy Clin Immunol. 2011 Jul;128(1):147-152.e2. doi: 10.1016/j.jaci.2011.04.037. Epub 2011 May 28. http://www.ncbi.nlm.nih.gov/pubmed/21621255 |
| Homann et al. 2010 | Homann J, Kolck UW, Ehnes A, Frieling T, Raithel M, Molderings GJ.: "Die systemische Mastozytose - Standortbestimmung einer internistischen Erkrankung [Systemic mastocytosis - definition of an internal disease]". Med Klin (Munich). 2010 Aug;105(8):544-53. Epub 2010 Sep 8. http://www.ncbi.nlm.nih.gov/pubmed/20824412 |
References and bibliography
The "back"-button of your browser takes you back to the previous position.
| A | Back to the previous position |
|---|---|
| Afrin 2014 | Lawrence B. Afrin: "The Bulk of the Iceberg revealed: Mast Cell Activation Syndrome". Gastvortrag vom 6. August 2014 an der University of Cape Town, Südafrika, ca. ab Minute 0:28:00 des Videos. http://meeting.uct.ac.za/p4j213xndbs/?launcher=false&fcsContent=true&pbMode=normal |
| B | Back to the previous position |
| Brockow 2013 | Prof. Dr. K. Brockow: "Mastzellaktivierungssyndrome". Der Hautarzt, February 2013, Volume 64, Issue 2, pp 102-106. http://link.springer.com/article/10.1007%2Fs00105-012-2452-6 |
| Brockow and Ring 2011 | Brockow K, Ring J.: "Update on diagnosis and treatment of mastocytosis". Curr Allergy Asthma Rep. 2011 Aug;11(4):292-9. http://www.ncbi.nlm.nih.gov/pubmed/21523372 |
| Broesby-Olsen et al. 2013 | Broesby-Olsen S1, Kristensen T, Vestergaard H, Brixen K, Møller MB, Bindslev-Jensen C: "KIT D816V mutation burden does not correlate to clinical manifestations of indolent systemic mastocytosis.". J Allergy Clin Immunol. 2013 Sep;132(3):723-8. doi: 10.1016/j.jaci.2013.02.019. Epub 2013 Apr 12. http://www.ncbi.nlm.nih.gov/pubmed/23587333 Die Mediatorkonzentrationen im Blut korrelieren nicht mit der Intensität der Symptome. |
| H | Back to the previous position |
| Haenisch et al. 2012 | Haenisch B1, Nöthen MM, Molderings GJ.: "Systemic mast cell activation disease: the role of molecular genetic alterations in pathogenesis, heritability and diagnostics." Immunology. 2012 Nov;137(3):197-205. doi: 10.1111/j.1365-2567.2012.03627.x. https://pubmed.ncbi.nlm.nih.gov/22957768 |
| Hamilton et al. 2011 | Hamilton MJ, Hornick JL, Akin C, Castells MC, Greenberger NJ.: "Mast cell activation syndrome: a newly recognized disorder with systemic clinical manifestations." J Allergy Clin Immunol. 2011 Jul;128(1):147-152.e2. doi: 10.1016/j.jaci.2011.04.037. Epub 2011 May 28. https://pubmed.ncbi.nlm.nih.gov/21621255 |
| Homann et al. 2010 | Homann J, Kolck UW, Ehnes A, Frieling T, Raithel M, Molderings GJ.: "Die systemische Mastozytose - Standortbestimmung einer internistischen Erkrankung [Systemic mastocytosis - definition of an internal disease]". Med Klin (Munich). 2010 Aug;105(8):544-53. Epub 2010 Sep 8. http://www.ncbi.nlm.nih.gov/pubmed/20824412 |
| J | Back to the previous position |
| Jarisch 2004 | Jarisch, Reinhart: "Histamin-Intoleranz, Histamin-Intoleranz und Seekrankheit", Thieme-Verlag, 2. Auflage, 2004. ISBN 3-13-105382-8 |
| K | Back to the previous position |
| Kofler et al. 2009 | H. Kofler, W. Aberer, M. Deibl, Th. Hawranek, G. Klein, N. Reider und N. Fellner: "Diaminoxidase keine diagnostische Hilfe bei Histaminintoleranz", Allergologie, vol. 32, no. 3, pp. 105–109, 2009. http://www.dustri.com/nc/de/deutschsprachige-zeitschriften/mag/allergologie/vol/jahrgang-32-3/issue/maumlrz-1.html (Nur Abstract kostenlos abrufbar) |
| Kofler et al. 2011 | Lukas Kofler, Hanno Ulmer, Heinz Kofler: "Histamine 50-Skin-Prick Test: A Tool to Diagnose Histamine Intolerance", ISRN AllergyVolume 2011 (2011), Article ID 353045, 5 pages. doi:10.5402/2011/353045. http://www.isrn.com/isrn/allergy/2011/353045/, abgerufen am 25.11.2011. |
| L | Back to the previous position |
| Lange et al. 2015 | Lange M, Lugowska-Umer H, Niedoszytko M, Wasag B, Limon J, Zawrocki A, Nedoszytko B, Sobjanek M, Plata-Nazar K, Nowicki R.: "Diagnosis of Mastocytosis in Children and Adults in Daily Clinical Practice." Acta Derm Venereol. 2015 Aug 13. doi: 10.2340/00015555-2210. https://pubmed.ncbi.nlm.nih.gov/26270728 Frei zugänglicher Artikel. "This comprehensive review presents currently defined variants of the disease and recommendations to facilitate diagnostic work-up in children and adults with suspected mastocytosis in daily clinical practice." |
| Lillestol et al. 2010 | Lillestøl K1, Helgeland L, Arslan Lied G, Florvaag E, Valeur J, Lind R, Berstad A.: "Indications for atopic bowel in patients with self-reported food hypersensitivity." Aliment Pharmacol Ther. 2010 May;31(10):1112-1122. doi: 10.1111/j.1365-2036.2010.04261.x. https://pubmed.ncbi.nlm.nih.gov/20163379 Seronegative gastrointestinale Nahrungsmittelallergien. |
| M | Back to the previous position |
| Maintz et al. 2006 | Maintz, Laura; Bieber, Thomas; Novak, Natalija: "Die verschiedenen Gesichter der Histaminintoleranz: Konsequenzen für die Praxis (Histamine Intolerance in Clinical Practice)", Deutsches Ärzteblatt 2006; 103(51-52). http://www.aerzteblatt.de/V4/archiv/artikel.asp?id=53958, abgerufen am 25.08.2009. |
| Molderings et al. 2014 | Molderings GJ, Homann J, Brettner S, Raithel M, Frieling T: "Systemische Mastzellaktivierungserkrankung: Ein praxisorientierter Leitfaden zu Diagnostik und Therapie" [Mast cell activation disease: a concise practical guide for diagnostic workup and therapeutic options]. Dtsch Med Wochenschr. 2014 Jul;139(30):1523-34; quiz 1535-8. doi: 10.1055/s-0034-1370055. Epub 2014 May 6. https://pubmed.ncbi.nlm.nih.gov/24801454 |
| Molderings et al. 2011 | Molderings GJ, Brettner S, Homann J, Afrin LB.: "Mast cell activation disease: a concise practical guide for diagnostic workup and therapeutic options". J Hematol Oncol. 2011 Mar 22;4:10. http://www.ncbi.nlm.nih.gov/pubmed/21418662 Frei zugänglicher Übersichtsartikel |
| R | Back to the previous position |
| Raithel et al. 2012 | Raithel, Martin; Hahn, Eckhart Georg; Baenkler, Hanns-Wolf: "Klinik und Diagnostik von Nahrungsmittelallergien: Gastrointestinal vermittelte Allergien Grad I bis IV." Dtsch Arztebl 2002; 99(12): A-780 / B-641 / C-599. http://www.aerzteblatt.de/archiv/30916/Klinik-und-Diagnostik-von-Nahrungsmittelallergien-Gastrointestinal-vermittelte-Allergien-Grad-I-bis-IV |
| Redaktion | Empfehlung des Redakteurs dieser Website oder des Autors dieser Seite, welche aus den Erfahrungen und Anschauungen von betroffenen Laien hervorgegangen ist und lediglich unseren aktuellen Stand des Unwissens widerspiegelt. |
| Reese et al. 2012 | Imke Reese, Barbara Ballmer-Weber, Kirsten Beyer, Stephan Erdmann, Thomas Fuchs, Jörg Kleinetebbe, Ludger Klimek, Ute Lepp, Margot Henzgen, Bodo Niggemann, Joachim Saloga, Christiane Schäfer, Thomas Werfel, Torsten Zuberbier, Margitta Worm: "Vorgehen bei Verdacht auf Unverträglichkeit gegenüber oral aufgenommenem Histamin. Leitlinie der Deutschen Gesellschaft für Allergologie und klinische Immunologie (DGAKI), der Gesellschaft für Pädiatrische Allergologie und Umweltmedizin (GPA) und des Ärzteverbandes Deutscher Allergologen (ÄDA)". AWMF 2012 http://dgaki.de/wp-content/uploads/2010/05/Leitlinie_Histaminunverträglichkeit2012.pdf (177 kb). http://www.awmf.org/uploads/tx_szleitlinien/061-030l_S1_Histaminunverträglichkeit_2012.pdf (177 kb). (Leitlinie zur Diagnose des oralen Histaminsyndroms. Konsensusdokument.) |
| V | Back to the previous position |
| Valent et al. 2012 | Valent P, Akin C, Arock M, Brockow K, Butterfield JH, Carter MC, Castells M, Escribano L, Hartmann K, Lieberman P, Nedoszytko B, Orfao A, Schwartz LB, Sotlar K, Sperr WR, Triggiani M, Valenta R, Horny HP, Metcalfe DD.: "Definitions, criteria and global classification of mast cell disorders with special reference to mast cell activation syndromes: a consensus proposal". Int Arch Allergy Immunol. 2012;157(3):215-25. Epub 2011 Oct 27. http://www.ncbi.nlm.nih.gov/pubmed/22041891 (Propose a global unifying classification of all MC disorders and pathologic MC reactions. This classification includes three types of 'MCA syndromes' (MCASs), namely primary MCAS, secondary MCAS and idiopathic MCAS. MCA is now defined by robust and generally applicable criteria, including (1) typical clinical symptoms, (2) a substantial transient increase in serum total tryptase level or an increase in other MC-derived mediators, such as histamine or prostaglandin D(2), or their urinary metabolites, and (3) a response of clinical symptoms to agents that attenuate the production or activities of MC mediators.) |
| Valent et al. 2007 | Valent P, Akin C, Escribano L, Födinger M, Hartmann K, Brockow K, Castells M, Sperr WR, Kluin-Nelemans HC, Hamdy NA, Lortholary O, Robyn J, van Doormaal J, Sotlar K, Hauswirth AW, Arock M, Hermine O, Hellmann A, Triggiani M, Niedoszytko M, Schwartz LB, Orfao A, Horny HP, Metcalfe DD.: "Standards and standardization in mastocytosis: consensus statements on diagnostics, treatment recommendations and response criteria." Eur J Clin Invest. 2007 Jun;37(6):435-53. https://pubmed.ncbi.nlm.nih.gov/17537151 Frei zugänglicher Übersichtsartikel |
| Vysniauskaite et al. 2015 | Vysniauskaite M, Hertfelder HJ, Oldenburg J, Dreßen P, Brettner S, Homann J, Molderings GJ: "Determination of plasma heparin level improves identification of systemic mast cell activation disease." PLoS One. 2015 Apr 24;10(4):e0124912. doi: 10.1371/journal.pone.0124912. eCollection 2015. https://pubmed.ncbi.nlm.nih.gov/25909362 Frei zugänglicher Artikel (Plasma heparin level appears more sensitive than the other mediators for detecting systemic MC activity in patients with MCAS. The simple, brief venous occlusion test appears to be a useful indicator of the presence of pathologically irritable MCs, at least in the obstructed compartment of the body.) |
| W | Back to the previous position |
| Wikipedia: gastrointestinale Lavage | Wikipedia: "Gastrointestinale Lavage" Wikipedia-Artikel, abgerufen am 29.6.2015. https://de.wikipedia.org/wiki/Gastrointestinale_Lavage Endoskopisch gesteuerte segmentale gastrointestinale Lavage, um lokale Allergien gegen Nahrungsmittelbestandteile zu bestimmen, deren Nachweis mit herkömmlichen Allergietests nicht immer gelingt. |
| Wöhrl et al. 2004 | Wöhrl S, Hemmer W, Focke M, Rappersberger K, Jarisch R.: "Histamine intolerance-like symptoms in healthy volunteers after oral provocation with liquid histamine.". Allergy Asthma Proc. 2004 Sep-Oct;25(5):305-11. Floridsdorf Allergy Center (FAZ), Vienna, Austria. http://www.ncbi.nlm.nih.gov/pubmed/15603203 (50% von zehn gesunden Frauen ohne Anzeichen von Nahrungsmittelunverträglichkeiten in der Vergangenheit reagierten in einer doppelblinden, placebokontrollierten Studie auf die Gabe von 75 mg Histamin in flüssiger Form mit Symptomen, während keine einzige Person auf das Placebo reagierte. Teilweise traten die Reaktionen zeitlich stark verzögert auf.) |
| Z | Back to the previous position |
| Zopf et al. 2009 | Zopf, Yurdagül; Baenkler, Hanns-Wolf; Silbermann, Andrea; Hahn, Eckhart G.;Raithel, Martin: "Differenzialdiagnose von Nahrungsmittelunverträglichkeiten / The Differential Diagnosis of Food Intolerance". Dtsch Arztebl Int 2009; 106(21): 359-69 |
![[Logo MCAD]](../pics/logo_mcas_160x160_bkg045552.png)